Tuesday, May 31, 2016
5.21 Understand that condensation polymerisation produces a small molecule, such as water, as well as the polymer.
When the two monomers come together, one or more atoms is/are lost. These join together to make a small molecule (i.e. H₂O, CH₃OH (methanol) or HCl). The monomers then join together to make the polymer.
5.20 Understand that some polymers, such as nylon, form by a different process called condensation polymerisation
Condensation polymerisation is a situation in which a polymer is formed, along with a small molecule such as H₂O, HCl or CH₃OH (methanol).
Nylon is an example:
Nylon is an example:
5.19 Explain that addition polymers are hard to dispose of as their inertness means that they do not easily biodegrade
Addition polymers are unreactive because they are saturated, which means they don't biodegrade easily. Solutions used today include:
- burning (not good, produces cancerous / harmful gases)
- landfills (takes up a lot of useful land and harmful chemicals leak into the soil)
- recycling (good for the environment but uses up energy and isn't always cheapest)
5.18 Describe some uses for polymers, including poly(ethene), poly(propene) and poly(chloroethene)
Poly(ethene)
- plastic bags
- light carrier bags
- plastic bottles
poly(propene)
- crates
- ropes
- thermal undergarments
poly(chloroethene)
- water pipes
- wire insulation
5.17 Deduce the structure of a monomer from the repeat unit of an addition polymer
In the simplest terms possible: take away the two 'floating' / empty bonds at the sides and make a carbon carbon double bond between 2 carbons instead.
5.16 Draw the repeat unit of addition polymers, including poly(ethene), poly(propene) and poly(chloroethene)
Repeat units must be drawn:
- within large brackets
- with two bonds sticking out of the brackets
- an n at the end, representing the number of times it is repeated (i.e. n could be anything, you don't write the number, write n)
Poly(ethene) and poly(chloroethene):
Poly(propene)
5.15 Understand that an addition polymer is formed by joining up many small molecules called monomers
Exactly what the point says: addition polymers are made by joining up many small ones called monomers: "mono" meaning one and "poly" meaning many
Monday, May 30, 2016
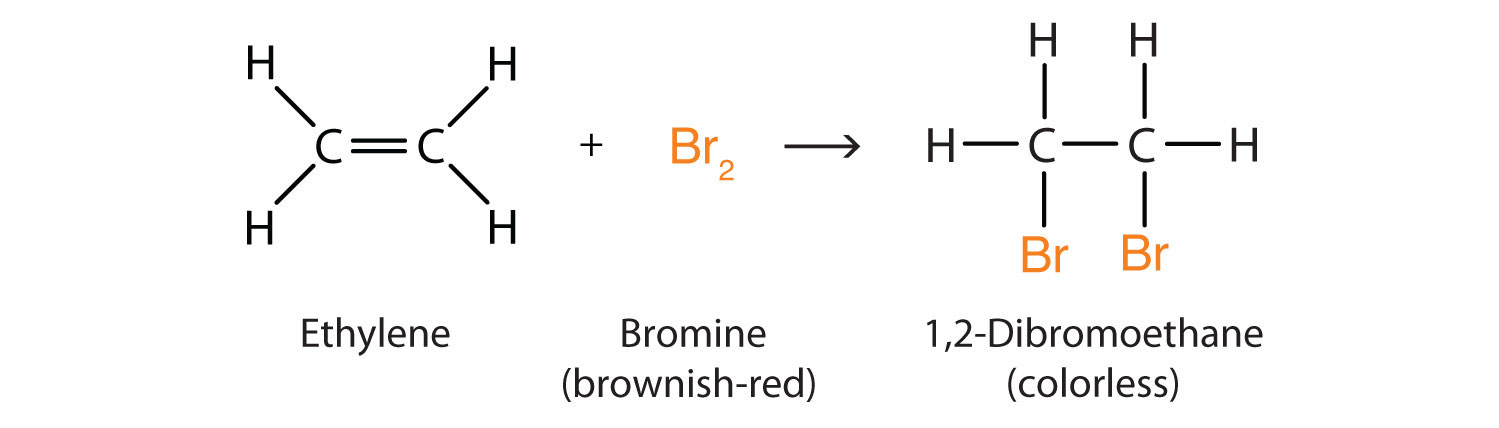
3.8 Describe the addition reaction of alkenes with bromine, including the decolourising of bromine water as a test for alkenes
When an alkene reacts with bromine, it turns one of its double (unsaturated) bonds into a single bond so it can bond with the bromine atoms. For example, ethane (shown below as ethylene) will bond with bromine to form 1,2 dibromoethane. (Br is on molecules 1 and 2, there are 2 of them, and the rest is ethane. That's the naming process aha)
Because of this, Br water will decolorize if you mix an alkane with it.
It will not decolorize immediately with alkenes because they are saturated.
Also, to bond to two bromines, the alkene must make its double bond a single bond, hence the name addition reaction
Sunday, May 29, 2016
1.55 Write ionic half-equations representing the reactions at the electrodes during electrolysis
At positive (anode) electrode, electrons are lost.
eg 2Br- > Br2 + 2e-
At negative (cathode) electrode, electrons are gained.
eg 2H+ + 2e- > H2
*Make sure charges are the same on both sides!!
eg 2Br- > Br2 + 2e-
At negative (cathode) electrode, electrons are gained.
eg 2H+ + 2e- > H2
*Make sure charges are the same on both sides!!
1.54 Describe experiments to investigate electrolysis, using inert electrodes, of aqueous solutions such as sodium chloride, copper(II) sulfate and dilute sulfuric acid and predict the products
- Place inert electrodes in aqueous ionic solution
- At positive electrode (anode), - ion will form an atom (non metal)
- At negative electrode (cathode), + ion will form an atom (metal)
eg
- Sodium chloride: sodium at cathode, chlorine at anode
- Copper(II) sulphate: copper at cathode, oxygen at anode, sulphur stays in solution
- Sulphuric acid: hydrogen at cathode, oxygen at anode, sulphur stays in solution
To help remember:
CINDY IS NOT AN INDECENT POTATO:
Cathode
Is
Negative
Anode
Is
Positive
NAP MAN:
Non-metal
At
Positive
Metal
At
Negative
CCOASS (for copper(II) sulphate)
(Cathy Chopped Apples And Stabbed Sue)
Copper
Cathode
Oxygen
Anode
Sulphur
Solution
HCOASS (for dilute sulphuric acid)
(Henry Cut Onions And Started Sulking)
Hydrogen
Cathode
Oxygen
Anode
Sulphur
Solution
1.53 Describe experiments to investigate electrolysis, using inert electrodes, of molten salts such as lead(II) bromide and predict the products
1.52 Understand that electrolysis involves the formation of new substances when ionic compounds conduct electricity
Ionic compounds conduct electricity when molten or in solution. During electrolysis, positively charged ions move to one side (cathode, which is the negatively charged electrode) and form metals. The negatively charged ions move to the other side (anode, the positively charged electrode) to form non metals. This is because opposite charges attract. These ions become atoms / new products because they undergo a reaction where either a gain or loss of electrons occurs.
TO REMEMBER:
Cathode
Is
Negative
Anode
Is
Positive
Cindy Is Not An Indecent Potato
TO REMEMBER:
Cathode
Is
Negative
Anode
Is
Positive
Cindy Is Not An Indecent Potato
1.51 Describe experiments to distinguish between electrolytes and non-electrolytes
- Set up a circuit with an LED light, a battery/power pack, wire and a solution
- Create a gap between the two ends of the wire. Place them in the solution.
- Switch the power pack on (if required)
- If LED lights up, a current is flowing through the molten substance / solution. This means it is an electrolyte.
- If it doesn't light up, there's no current, therefore it is not an electrolyte.
Or you could just...
1.50 Understand why ionic compounds conduct electricity only when molten or in solution
When in solution, ions separate (to form + and - ions, accordingly) and they become free to move, so they can carry electricity and so the compound can now conduct when it is molten / in solution.
1.49 Understand why covalent compounds do not conduct electricity
In covalent substances, the electrons are not free to move, so current can't move through the substance; there's no electrons to carry it - no transfer of electricity.
1.48 Understand that an electric current is a flow of electrons or ions
Surprise! An electric current is a flow of electrons or ions. Well done, me.
Saturday, May 28, 2016
5.14 describe how long-chain alkanes are converted to alkenes and shorter-chain alkanes by catalytic cracking, using silica or alumina as the catalyst and a temperature in the range of 600–700ºC
Passing long-chain hydrocarbons over a hot catalyst (in this case silica or alumina at roughly 600-700ºC) will cause them to break down into smaller chains of hydrocarbons
Some of the atoms are lost from the molecules, making them unsaturated and able to form a double bond. This is how you can get alkenes from cracking, not just short-chain hydrocarbons
Some of the atoms are lost from the molecules, making them unsaturated and able to form a double bond. This is how you can get alkenes from cracking, not just short-chain hydrocarbons
5.13 Understand that fractional distillation of crude oil produces more long-chain hydrocarbons than can be used directly and fewer short-chain hydrocarbons than required and explain why this makes cracking necessary
The main problem is that long-chain hydrocarbons are more viscous and less flammable, whereas the short-chain hydrocarbons flow and burn well, which makes them more useful.
However, as the title said, more long-chain hydrocarbons are produced then short-chain ones.
TADA! We have cracking, which breaks up the longer, less useful hydrocarbons into shorter, more useful hydrocarbon
However, as the title said, more long-chain hydrocarbons are produced then short-chain ones.
TADA! We have cracking, which breaks up the longer, less useful hydrocarbons into shorter, more useful hydrocarbon
5.12 Understand that nitrogen oxides and sulfur dioxide are pollutant gases which contribute to acid rain, and describe the problems caused by acid rain
NO (nitrogen oxide) is produced in car engines.
When nitrogen oxide and sulfur dioxide are in the atmosphere they react with rainwater to create H+ ions, making it more acidic. This means that when rain falls it can alter the PH in soil or rivers which will severely affect the ecosystem. It can also corrode limestone, damaging rocks, buildings (a big problem if the buildings are historical ones)
When nitrogen oxide and sulfur dioxide are in the atmosphere they react with rainwater to create H+ ions, making it more acidic. This means that when rain falls it can alter the PH in soil or rivers which will severely affect the ecosystem. It can also corrode limestone, damaging rocks, buildings (a big problem if the buildings are historical ones)
5.11 Understand that, in car engines, the temperature reached is high enough to allow nitrogen and oxygen from air to react, forming nitrogen oxides
Literally what it says in the title.
In car engines, the temperature is high enough for nitrogen and oxygen in the air to react, forming NO
In car engines, the temperature is high enough for nitrogen and oxygen in the air to react, forming NO
5.10 Understand that incomplete combustion of fuels may produce carbon monoxide and explain that carbon monoxide is poisonous because it reduces the capacity of the blood to carry oxygen
Usually hydrogens combust with oxygen to give carbon dioxide and and water, like so:
- Hydrocarbons + oxygen > carbon dioxide + water
- Hydrocarbons + oxygen > carbon monoxide + carbon + water
5.9 Describe the trend in boiling point and viscosity of the main fractions
Fractions with low boiling points are less viscous than fractions with higher boiling points.
5.7 Describe and explain how the industrial process of fractional distillation separates crude oil into fractions
Crude oil is separated into it's various hydrocarbons by fractional distillation, which happens like so:
1. Crude oil is heated
2. As a gas it floats upwards, where the temperature decreases
3. One by one, each compound in the crude oil will reach it's condensing point and condense
4. It will then be collected
This ensures that all groups with similar condensing temperatures (known as fractions) are each separated, since each fraction is a different substance
1. Crude oil is heated
2. As a gas it floats upwards, where the temperature decreases
3. One by one, each compound in the crude oil will reach it's condensing point and condense
4. It will then be collected
This ensures that all groups with similar condensing temperatures (known as fractions) are each separated, since each fraction is a different substance
5.6 Understand that crude oil is a mixture of hydrocarbons
Basically, crude oil is made up of different hydrocarbons (molecules with ONLY hydrogen and carbon atoms in them)
3.12 Describe the dehydration of ethanol to ethene, using aluminium oxide.
- Ethanol > ethene + water
- C2H5OH > C2H4 + H2O
3.11 Evaluate the factors relevant to the choice of method used in the manufacture of ethanol, for example the relative availability of sugar cane and crude oil
HYDRATION OF ETHENE
- Pros
- The process is continuous
- Only one product is produced (no need to separate them afterwards)
- Fewer workers are needed
- Fast process
- Cons
- Comes from non-renewable, expensive sources (crude oil, cracked to make ethene)
- High temperature and pressure is needed
- A lot of energy is used
- Pros
- Sugar is a renewable source
- It is also widely available and cheap
- Normal pressure and a warm environment used
- Cons
- Two products are produce (they are impure and need to be purified)
- It is a batch process (stop-start)
- A lot of workers are needed
- It is a slow process
3.10 Describe the manufacture of ethanol by the fermentation of sugars, for example glucose, at a temperature of about 30°C
The (natural) catalyst for this reaction are the enzymes found in single-celled fungi (yeast)
The anaerobic respiration of microorganisms can produce ethanol.
The reaction also takes place at a temperature of 30°C
The absence of air is required for this reaction, and the products are purified by fractional distillation
The anaerobic respiration of microorganisms can produce ethanol.
- Glucose > ethanol + carbon dioxide
- C66H12O6 > 2C2H5OH + 2CO2
The reaction also takes place at a temperature of 30°C
The absence of air is required for this reaction, and the products are purified by fractional distillation
3.9 Describe the manufacture of ethanol by passing ethene and steam over a phosphoric acid catalyst at a temperature of about 300°C and a pressure of about 60–70 atm
Ethanol can be made by reacting ethene (from cracking crude oil fractions) with steam (aka the hydration of ethene).
- The pressure (60-70 atm) at which the reaction takes place
- The temperature (300°C)
The process is continuous (as long as there are always reactants)
The high pressure and temperature make the reaction happen really quickly. This is described as the dehydration of ethene (I'm pretty sure)
- Ethene + steam > ethanol
- C2H4 + H2O > C2H5OH
- The pressure (60-70 atm) at which the reaction takes place
- The temperature (300°C)
The process is continuous (as long as there are always reactants)
The high pressure and temperature make the reaction happen really quickly. This is described as the dehydration of ethene (I'm pretty sure)
3.7 Draw displayed formulae for alkenes with up to four carbon atoms in a molecule, and name the straight-chain isomers
3.6 Recall that alkenes have the general formula CnH2n
Basically, all compounds in the homologous group alkenes have the general formula CnH2n, which means that for every carbon atom there are two hydrogen ones
3.5 Describe the substitution reaction of methane with bromine to form bromomethane in the presence of UV light.
(Taken directly, word for word (because I am extremely lazy right now) from Hannah Help Chemistry)
In UV light bromine and methane will form bromomethane:
CH4 + Br2 >CH3Br + HBr
What has happened in this reaction is a bromine has taken the place of a hydrogen (substitution.)
In UV light bromine and methane will form bromomethane:
CH4 + Br2 >CH3Br + HBr
3.4 Recall the products of the complete and incomplete combustion of alkanes
Complete combustion of alkanes will give you carbon dioxide and water
Incomplete combustion will give you carbon monoxide and water
Incomplete combustion will give you carbon monoxide and water
Friday, May 27, 2016
3.3 Draw displayed formulae for alkanes with up to five carbon atoms in a molecule, and name the straight-chain isomers
All you need to remember when drawing displayed formulas for alkanes is that every carbon is bonded to two hydrogens (usually draw them one on top and one below) and there are two hydrogens on the end of the whole thing.
Below is the displayed formula for
1. Methane

2. Ethane

3. Propane

4. Butane

5. Pentane

Below is the displayed formula for
1. Methane

2. Ethane

3. Propane

4. Butane

5. Pentane

1.23 Understand how the formulae of simple compounds can be obtained experimentally, including metal oxides, water and salts containing water of crystallisation
- Weigh the compound
- Remove one element of it through a reaction
- Weigh again
- If the first weight [mass] of the compound is AB and the second weight [mass] is A, you can work out B by: AB-A=B
- Work it out by dividing the weight by the relative atomic mass
Thursday, May 26, 2016
3.2 Recall that alkanes have the general formula CnH2n+2
If you see the post tagged 3.1, a general formula is a formula that all members of a homologous series (in this case, the alkanes) will fit.
Basically (in every single alkane) for every carbon atom there will be twice the amount of hydrogen plus two.
- E.g.: 2 carbons
- 2(2) + 2 hydrogens = 6 hydrogens
Basically (in every single alkane) for every carbon atom there will be twice the amount of hydrogen plus two.
- E.g.: 2 carbons
- 2(2) + 2 hydrogens = 6 hydrogens
Monday, May 23, 2016
3.1 Explain the terms homologous series, hydrocarbon, saturated, unsaturated, general formula and isomerism.
HOMOLOGOUS SERIES:
"A homologous series is a family of hydrocarbons with similar chemical properties who share the same general formula" (BBC Bitesize). Basically, compounds (in this case hydrocarbons) in the same homologous series have the SAME general formula and SIMILAR chemical properties
HYDROCARBON
A compounds made up of ONLY hydrogen and carbon atoms
SATURATED
A compound is described as 'saturated' if it ONLY has single bonds present. This means that the compounds has "bonded as many times as possible" (Hannah Help).
UNSATURATED
A hydrocarbon with a double carbon carbon bond is described as unsaturated, because more bonds can be made (as can be seen later in polymerisation)
GENERAL FORMULA
A formula that all members of the same homologous series will fit.
ISOMERISM
Compounds with the same chemical formulas, but different structural formulas
"A homologous series is a family of hydrocarbons with similar chemical properties who share the same general formula" (BBC Bitesize). Basically, compounds (in this case hydrocarbons) in the same homologous series have the SAME general formula and SIMILAR chemical properties
HYDROCARBON
A compounds made up of ONLY hydrogen and carbon atoms
SATURATED
A compound is described as 'saturated' if it ONLY has single bonds present. This means that the compounds has "bonded as many times as possible" (Hannah Help).
UNSATURATED
A hydrocarbon with a double carbon carbon bond is described as unsaturated, because more bonds can be made (as can be seen later in polymerisation)
GENERAL FORMULA
A formula that all members of the same homologous series will fit.
ISOMERISM
Compounds with the same chemical formulas, but different structural formulas
Sunday, May 22, 2016
2.38 Describe tests for anions
i) USING DILUTE NITRIC ACID & SILVER NITRATE SOLUTION (depending on the precipitate)
- Chloride ions (Cl-) + nitric acid + silver nitrate > white precipitate
- Precipitate = silver chloride
- Bromide ions + nitric acid + silver nitrate > cream precipitate
- Precipitate = silver bromide
- Iodide ions + nitric acid + silver nitrate > yellow precipitate
- Precipitate = silver iodide
ii) USING DILUTE HYDROCHLORIC ACID & BARIUM CHLORIDE SOLUTION
- Sulphate ions + hydrochloric acid + barium chloride > white precipitate
- SO4(2-) + HCl + BaCl2 (2+) > BaSO4
- Precipitate = barium sulphate
iii) USING DILUTE HYDROCHLORIC ACID (& identifying if CO2 is produced)
- Carbonate + hydrochloric acid > salt + water + carbon dioxide
- Test for CO2:
- When bubbled through lime water, will turn it cloudy
Friday, May 20, 2016
2.37 Describe tests for the following cations:
i) FLAME TESTS
- Li+ (Lithium) burns with a red flame
- Na+ (Sodium) burns with a strong orange flame (sometimes it is so strong it can mask other colours)
- K+ (Potassium) lilac flame
- Ca2+ (Calcium) burns with a brick red flame
ii) NH4+ USING SODIUM HYDROXIDE SOLUTION (& identifying the ammonia produced)
- ammonium ions + hydroxide ions > ammonia + water
- (NH4 + OH > NH3 + H2O)
- How to test for ammonia:
- ammonia will turn red litmus paper blue
- it also has a 'pungent' smell
iii) USING SODIUM HYDROXIDE SOLUTION (CU2+, FE2+, FE3+)
- Copper (ii) sulphate + sodium hydroxide > blue precipitate
- CuSO4 + NaOH
- Iron (ii) sulphate + sodium hydroxide > green precipitate
- FeSO4 + NaOH
- Iron (iii) sulphate + sodium hydroxide > brown precipitate
- Fe2(SO4)3 + NaOH
Wednesday, March 2, 2016
5.1 Explain how the methods of extraction if the metals in this section are related to their positions in the reactivity series
5.3 Write ionic half-equations for the reactions at the electrodes in aluminium extraction
These are the two equations you need to know (all thanks go to Hannah Help)
Al3+ + 3e- > Al
Al3+ + 3e- > Al
- 2O2- → O2 + 4e-
(btw, the Al and the O2 come from the aluminium oxide which is broken down via electrolysis)
5.5 Explain the uses of aluminium and iron, in terms of their properties
Aluminium:
It has low density, which makes it light for its size, and it is strong. This makes it good for the bodies of planes, light vehicles and ladders.
Also, it has a very thin layer of its oxides in its surface, preventing water and oxygen getting to it, and therefore making aluminium a metal which resists oxidation. Aluminium is also malleable (like most metals), making it ideal for drinking cans and cooking foil.
Finally, it conducts heat and electricity well (although not as well as copper, so maybe don't make this your first choice of property) so it is used in power cables and saucepans (and other cooking materials).
Iron:
Iron is one of the three magnetic materials (the other being cobalt and nickel) so it is usually used in electromagnets (for example as a huge electromagnet in scrapyards which picks up cars and other magnetic materials).
There are two types of iron:
- WROUGHT IRON (aka pure iron): malleable and soft, and mainly used as ornamental work in gates)
- CAST IRON: an alloy of iron and carbon (NOT steel). It is very brittle, but has a greater resistance to corrosion than wrought iron or steel and is also VERY STRONG (used for manhole covers on roads and pavements and as engine blocks for petrol/diesel engines)
It has low density, which makes it light for its size, and it is strong. This makes it good for the bodies of planes, light vehicles and ladders.
Also, it has a very thin layer of its oxides in its surface, preventing water and oxygen getting to it, and therefore making aluminium a metal which resists oxidation. Aluminium is also malleable (like most metals), making it ideal for drinking cans and cooking foil.
Finally, it conducts heat and electricity well (although not as well as copper, so maybe don't make this your first choice of property) so it is used in power cables and saucepans (and other cooking materials).
Iron:
Iron is one of the three magnetic materials (the other being cobalt and nickel) so it is usually used in electromagnets (for example as a huge electromagnet in scrapyards which picks up cars and other magnetic materials).
There are two types of iron:
- WROUGHT IRON (aka pure iron): malleable and soft, and mainly used as ornamental work in gates)
- CAST IRON: an alloy of iron and carbon (NOT steel). It is very brittle, but has a greater resistance to corrosion than wrought iron or steel and is also VERY STRONG (used for manhole covers on roads and pavements and as engine blocks for petrol/diesel engines)
- Iron can be used to form an alloy called steel which is often used in construction
- Used to make magnets
- Used to make boats, cars, etc.
- Used to make surgical equipment
(more uses click here)
See the BBC Bitesize page - it's really helpful
(http://www.bbc.co.uk/schools/gcsebitesize/science/aqa_pre_2011/rocks/metalsrev2.shtml)
Tuesday, March 1, 2016
5.4 Describe and explain the main reactions involved in the extraction of iron from iron ore (haematite), using coke, limestone and air in a blast furnace
Figure 1: All the equations you need to know
Figure 2
Coke, which contains carbon, burns in air to produce heat (and CO2), meaning that it is exothermic. The CO2 reacts with MORE C to form carbon monoxide, which is needed to reduce iron oxide.
Limestone, which contains calcium carbonate, is used to get rid of impurities: it reacts with them to produce molten slag, which can be used to build roads.
Hot air is needed so the coke can burn.
Because carbon is higher up in the reactivity series than iron, it displaces iron in the reaction Fe2O3 + 3CO → 2Fe + 3CO2
In that reaction, iron oxide is reduced to iron and carbon monoxide is oxidised to produce carbon dioxide.
5.2 Describe and explain the extraction of aluminium from PURIFIED aluminium oxide by electrolysis..
..including:
i. the use of molten cryolite as a solvent and to decrease the required operating temperature
ii. the need to replace positive electrodes
iii. the cost of electricity as a major factor
The extraction
1. Bauxite purified into aluminium oxide
2. Dissolved in molten cryolite - this is an aluminium compound and brings down the boiling point (less electricity needed = less energy needed = less cost). Also a solvent for Al2O3
3. At the negative electrode, aluminium is formed.
4. Oxygen forms at the positive electrode
5. Oxygen reacts with the positive graphite anode and it becomes corroded so it needs to be replaced frequently.
The electricity needed in this process costs a lot of money, so the process tries to use as little electricity as possible (which is a lot, to be honest)
Little key notes
i. the use of molten cryolite as a solvent and to decrease the required operating temperature
ii. the need to replace positive electrodes
iii. the cost of electricity as a major factor
The extraction
1. Bauxite purified into aluminium oxide
2. Dissolved in molten cryolite - this is an aluminium compound and brings down the boiling point (less electricity needed = less energy needed = less cost). Also a solvent for Al2O3
3. At the negative electrode, aluminium is formed.
4. Oxygen forms at the positive electrode
5. Oxygen reacts with the positive graphite anode and it becomes corroded so it needs to be replaced frequently.
The electricity needed in this process costs a lot of money, so the process tries to use as little electricity as possible (which is a lot, to be honest)
Little key notes
- Formula for aluminium oxide: Al2 O3 (numbers in subscript)
- Equation for this is 2Al2 O3 (l) ---> 4Al (l) + 3O2 (g) (numbers in red are used to balance the equation)
- It forms the ions Al 3+ (which is the cathode [-] ) and O 2- (which is the anode [+])
- Aluminium normally melts at about 2000 degrees C but in cryolite, it melts at about 900 degrees C
- Uses include aircraft construction (it's low density + strong), cans for drinks (easy to shape + corrosion resistant) and boilers and cookers (good heat conductor)
- An ionic compound will only be able to conduct electricity if it is molten or in an aqueous solution.
- Walls of the thingie (see below) are the negative anodes.
- For electricity to flow, there must be either delocalized electrons or ions that are able to move.
Here's a little diagram I drew that looks kind of depressing but hey, I tried.
Figure 1
See key word quizlet - link on the right hand side menu.
Subscribe to:
Posts (Atom)
.jpg)

%20bromide%20image%201.JPG)







