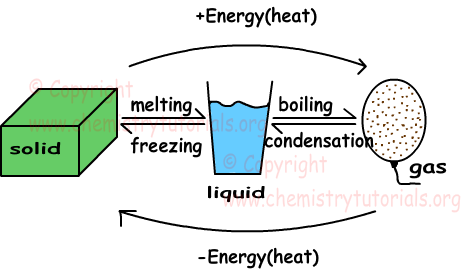
 |
| Figure 1 |
- Solid → Liquid = melting
- Liquid → Solid = freezing
- Liquid → Gas = boiling
- Gas → Liquid = condensing
- Solid → Gas = subliming
THE REASON LIQUID to GAS IS BOILING AND NOT EVAPORATING:
Because when its evaporating only the particles at the top are gaining energy and so becoming a gas. When its boiling all the particles are gaining energy
How it works:
- When heat is added, the particles begin to move faster until they move so much that the forces of attraction are no longer strong enough to hold them. This is when the change of state takes place.
- When heat is "removed", the process happens in reverse. The particles begin to lose energy, and move slower. The forces of attraction become strong enough to pull the particles closer to each other and hold them there.
Products listed on our website are either in stock or can be resynthesized within a reasonable time frame. 1-hexyl-3-methylimidazolium perfluorobutanesulfonate
ReplyDelete