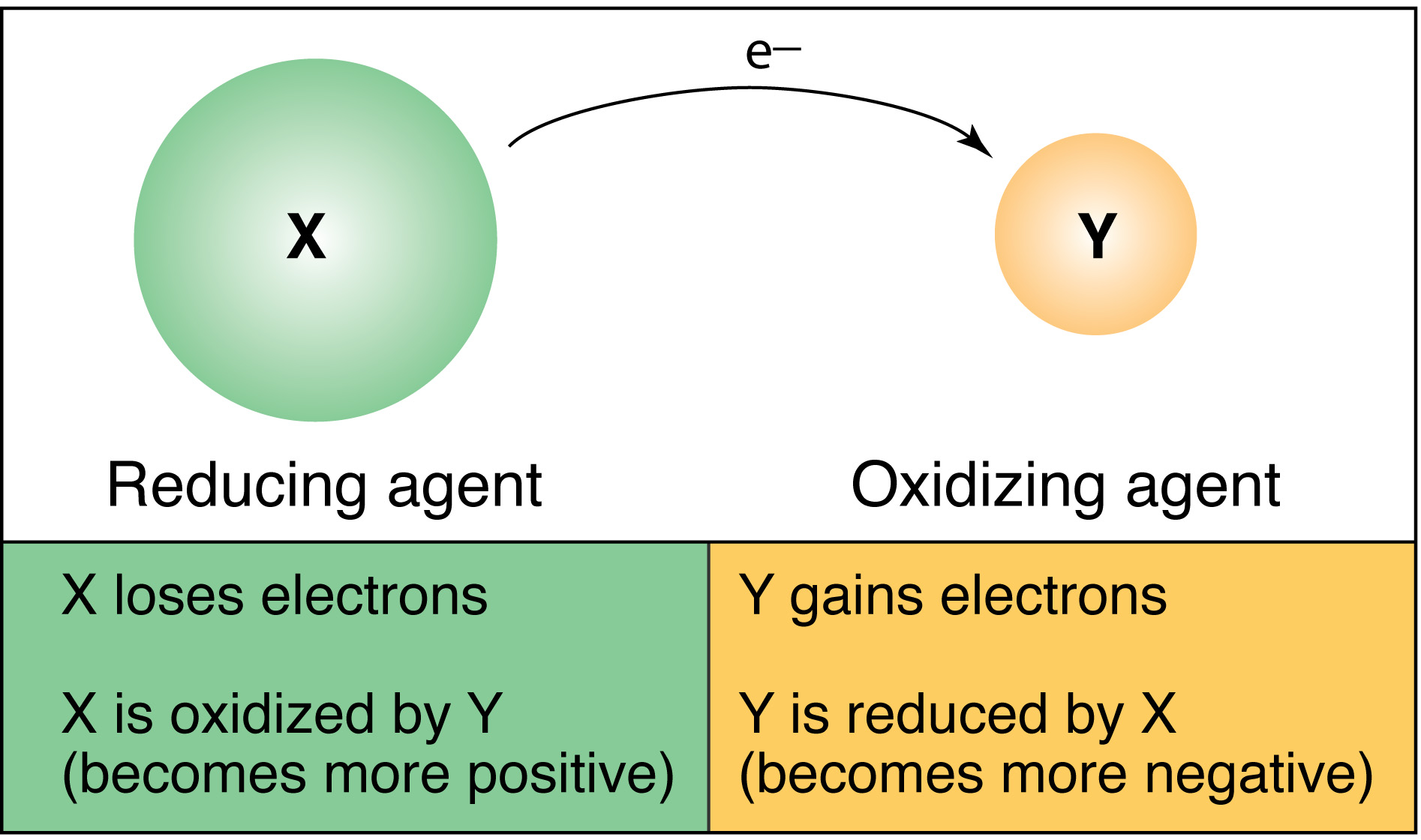
When a more reactive halogen displaces a less reactive halogen, it's called a redox reaction. This is because an element has gained something and the other has lost something:
OIL RIG:
Oxidisation
Is
Loss (of electrons)
Reduction
Is
Gain (of electrons)
No comments:
Post a Comment