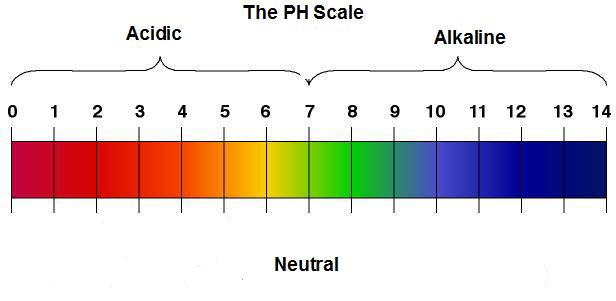
- Solutions with a pH less than 7 are acidic
- with 0 being a very strong (the strongest, really) acid
- and 6 (or as the number is closer to 7) being a very weak acid
- Solutions with a pH of 7 are neutral
- Solutions with a pH greater than 7 are alkaline
- where 8 (or as the number gets closer to 7) is a very weak alkali
- and 14 is a very strong (the strongest) alkali
Friday, November 13, 2015
4.2 Understand how the pH scale, from 0–14, can be used to classify solutions as strongly acidic, weakly acidic, neutral, weakly alkaline or strongly alkaline
The ph scale is used to measure acidity and alkilinity
Labels:
4.2,
acids and alkalis
Subscribe to:
Post Comments (Atom)
No comments:
Post a Comment